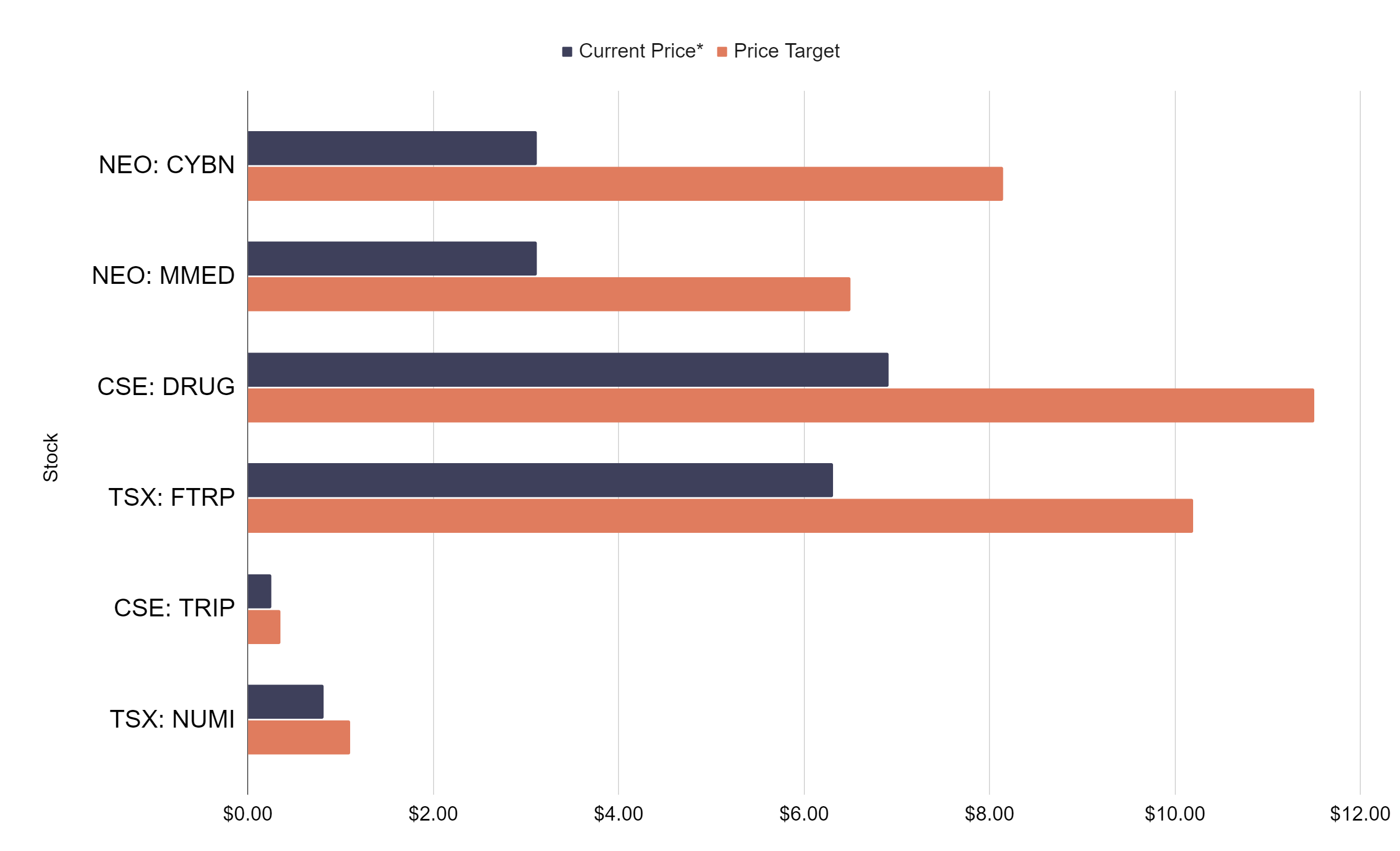
Cybin Announces Additional Adelia Milestone Achievements
TORONTO–(BUSINESS WIRE)– Cybin Inc. (NEO:CYBN) (NYSE AMERICAN:CYBN) (“Cybin” or the “Company”), a biotechnology company focused on psychedelic pharmaceutical therapies, is pleased to announce that Adelia Therapeutics Inc. (“Adelia”), a wholly-controlled subsidiary of Cybin, has achieved those milestones identified as Year 2 Q1 (i)-(iii), as contemplated by the terms of a contribution agreement dated December 4, 2020 (the “Transaction Agreement”) among Cybin, Cybin Corp., Cybin US Holdings Inc. (the “Acquiror”), a wholly-controlled subsidiary of Cybin, and all of the previous shareholders of Adelia (the “Adelia Shareholders”).
Pursuant to the terms of the Transaction Agreement, Class B common shares in the capital of the Acquiror (the “Class B Shares”) shall be issued to the Adelia Shareholders, in satisfaction of the $706,586.69 (approximately US$560,181.93) due to them on meeting a portion of the relevant milestones, at an effective issue price determined in accordance with the Transaction Agreement and applicable securities law. The Class B Shares issued by the Acquiror to the Adelia Shareholders are exchangeable for common shares in the capital of Cybin (the “Cybin Shares”) on a 10 Cybin Shares for 1 Class B Share basis, at the option of the holder thereof, subject to customary adjustments. No Class B Shares are exchangeable prior to December 14, 2021, and not more than: (i) 33 1/3% of the Class B Shares will be exchangeable prior to December 14, 2022; (ii) 66 2/3% of the Class B Shares will be exchangeable prior to December 14, 2023; and (iii) thereafter, 100% of the Class B Shares will be exchangeable.
Additional information related to the transaction is available in the Transaction Agreement, which is filed under Cybin’s profile on SEDAR (www.sedar.com) and with the U.S. Securities and Exchange Commission on EDGAR at www.sec.gov.
About Cybin
Cybin is a leading biotechnology company focused on researching and progressing psychedelic therapeutics by utilizing proprietary drug discovery platforms, innovative drug delivery systems, novel formulation approaches and potential treatment regimens for psychiatric disorders.
About Adelia
Adelia is a wholly-controlled subsidiary of the Company, that aims to develop medicinal psychedelics with improved dosing efficacy and therapeutic indices to address unmet medical needs. Adelia’s primary focus is on the development of treatment regimens consisting of proprietary psychedelic molecules and related clinical protocols. This proprietary development strategy is based on chemical modifications to the known and well understood tryptamine derivatives that significantly modify their pharmacokinetic properties without changing their therapeutic potential. These proprietary approaches seek to minimize inter-patient variability by better controlling drug metabolism without loss of efficacy that together have been shown to produce more predictable and favorable patient outcomes.
Cautionary Notes and Forward-Looking Statements
Certain statements in this press release constitute forward-looking information. All statements other than statements of historical fact contained in this press release, including, without limitation, statements regarding Cybin’s future, strategy, plans, objectives, goals and targets, and any statements preceded by, followed by or that include the words “believe”, “expect”, “aim”, “intend”, “plan”, “continue”, “will”, “may”, “would”, “anticipate”, “estimate”, “forecast”, “predict”, “project”, “seek”, “should” or similar expressions or the negative thereof, are forward-looking statements. Forward looking statements in this news release include statements regarding the Company’s development of innovative drug delivery systems, novel formulation approaches and potential treatment regimens for psychiatric disorders and Adelia’s proprietary development strategy and development of medicinal psychedelics with improved dosing efficacy and therapeutic indices to address unmet medical needs.
These forward-looking statements are based on reasonable assumptions and estimates of management of the Company at the time such statements were made. Actual future results may differ materially as forward-looking statements involve known and unknown risks, uncertainties, and other factors which may cause the actual results, performance, or achievements of the Company to materially differ from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors, among other things, include: implications of the COVID-19 pandemic on the Company’s operations; fluctuations in general macroeconomic conditions; fluctuations in securities markets; expectations regarding the size of the psychedelics market; the ability of the Company to successfully achieve its business objectives; plans for growth; political, social and environmental uncertainties; employee relations; the presence of laws and regulations that may impose restrictions in the markets where the Company operates; and the risk factors set out in the Company’s management’s discussion and analysis for the three months ended June 30, 2021 and the Company’s listing statement dated November 9, 2020, which are available under the Company’s profile on www.sedar.com and with the U.S. Securities and Exchange Commission on EDGAR at www.sec.gov. Although the forward-looking statements contained in this news release are based upon what management of the Company believes, or believed at the time, to be reasonable assumptions, the Company cannot assure shareholders that actual results will be consistent with such forward-looking statements, as there may be other factors that cause results not to be as anticipated, estimated or intended. Readers should not place undue reliance on the forward-looking statements and information contained in this news release. The Company assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change, except as required by law.
Cybin makes no medical, treatment or health benefit claims about Cybin’s proposed products. The U.S. Food and Drug Administration, Health Canada or other similar regulatory authorities have not evaluated claims regarding psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds or nutraceutical products. The efficacy of such products has not been confirmed by approved research. There is no assurance that the use of psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds or nutraceuticals can diagnose, treat, cure or prevent any disease or condition. Vigorous scientific research and clinical trials are needed. Cybin has not conducted clinical trials for the use of its proposed products. Any references to quality, consistency, efficacy and safety of potential products do not imply that Cybin verified such in clinical trials or that Cybin will complete such trials. If Cybin cannot obtain the approvals or research necessary to commercialize its business, it may have a material adverse effect on Cybin’s performance and operations.
The Neo Exchange Inc. has neither approved nor disapproved the contents of this news release and is not responsible for the adequacy and accuracy of the contents herein.
Unless otherwise indicated, all dollar amounts in this news release are expressed in Canadian dollars.
Investors:
Tim Regan/Scott Eckstein
KCSA Strategic Communications
Cybin@kcsa.com
Lisa M. Wilson
In-Site Communications, Inc.
lwilson@insitecony.com
Media:
John Kanakis
Cybin Inc.
John@cybin.com
Source: Cybin Inc.