AbbVie Announces First Provincial Reimbursement for VENCLEXTA® (venetoclax) with Obinutuzumab for Patients with Previously Untreated Chronic Lymphocytic Leukemia in Quebec
- Following the signing of an agreement between AbbVie and the pan-Canadian Pharmaceutical Alliance (pCPA), Quebec is the first province to reimburse the combination treatment.
- VENCLEXTA® (venetoclax) plus obinutuzumab is the first chemotherapy-free, fixed-duration combination regimen approved by Health Canada for patients with previously untreated chronic lymphocytic leukemia (CLL).
MONTREAL, Nov. 18, 2021 /CNW/ – AbbVie (NYSE: ABBV), a research-based global biopharmaceutical company, announced today that an agreement has been reached with the pan-Canadian Pharmaceutical Alliance (pCPA) for VENCLEXTA® (venetoclax) in combination with obinutuzumab for the treatment of adult patients with previously untreated chronic lymphocytic leukemia (CLL). The regimen combines six 28-day cycles of obinutuzumab with 12 cycles of VENCLEXTA.1
Effective November 10, on Québec’s Listes des médicaments, VENCLEXTA is listed in combination with obinutuzumab, for the treatment of adult patients with previously untreated chronic lymphocytic leukemia. For full criteria, consult the list of medications in effect.2
“Being able to prescribe a therapy, such as venetoclax in combination with obinutuzumab, that has a finite treatment duration and manageable side effects is a welcomed option for my patients. After completing the prescribed treatment regimen, I can tell them that they can stop their medication because this effective combination helps to delay disease progression,” explains Dr. Sarit Assouline, Physician, Division of Hematology, Sir Mortimer B. Davis-Jewish General Hospital, Senior Investigator, Lady Davis Institute for Medical Research, Associate Professor, Department of Oncology, McGill University.
In October 2020, the Institut national d’excellence en santé et en services sociaux (INESSS) recommended that the Minister include VENCLEXTA, in combination with obinutuzumab, on the Listes des médicaments for the treatment of CLL. INESSS concluded that “the results of the intermediate analysis show that the venetoclax/obinutuzumab combination as first-line therapy in patients with CLL prolongs progression-free survival in a statistically and clinically meaningful way compared to the chlorambucil/obinutuzumab combination.”3
“Lymphoma Canada is pleased that this combination is available on the Listes des médicaments in Québec for the treatment of chronic lymphocytic leukemia. Due to the nature of the disease and its high relapse rate, it is important to offer patients effective treatment options so that they can face their cancer journey with the comfort of knowing that there are always alternatives,” says Antonella Rizza, Chief Executive Officer, Lymphoma Canada.
VENCLEXTA, in combination with obinutuzumab, is the third indication for VENCLEXTA for the treatment of CLL, a first-in-class B-cell lymphoma-2 (BCL-2) inhibitor. BCL-2 is a protein that prevents cancer cells from undergoing apoptosis, the process that leads to the natural death or self-destruction of cancer cells. VENCLEXTA is also approved in combination with rituximab for the treatment of adult patients with CLL who have received at least one prior therapy, and as a monotherapy for the treatment of patients with CLL with 17p deletion who have received at least one prior therapy, or patients with CLL without 17p deletion who have received at least one prior therapy and for whom there are no other available treatment options.1
“We are very proud of this tremendous milestone bringing VENCLEXTA plus obinutuzumab to people living with CLL in Quebec. This combination is a much-needed treatment option and reinforces our commitment to people living with blood cancer,” says Tracey Ramsay, Vice President and General Manager, AbbVie Canada.
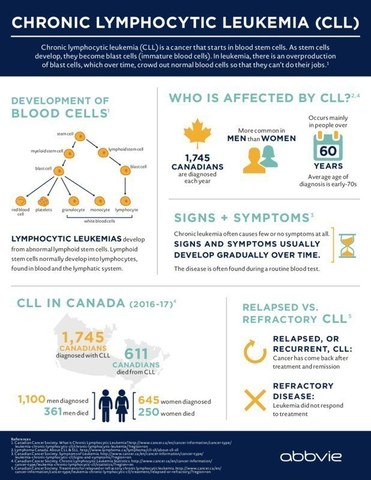
CLL, which is typically a slow-progressing cancer of the bone marrow and blood[4], is one of the most common types of leukemia in adults. In Canada, CLL accounts for approximately 1,745 newly diagnosed cases of leukemia each year and is responsible for more than 600 deaths a year.5
VENCLEXTA is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the U.S. and by AbbVie outside of the U.S.
About AbbVie in Oncology
At AbbVie, we strive to discover and develop medicines that deliver transformational improvements in cancer treatment by uniquely combining our deep knowledge in core areas of biology with cutting-edge technologies, and by working together with our partners – scientists, clinical experts, industry peers, advocates, and patients. We remain focused on delivering these transformative advances in treatment across some of the most debilitating and widespread cancers. We are also committed to exploring solutions to help patients obtain access to our cancer medicines. AbbVie’s oncology portfolio consists of marketed medicines and a robust pipeline containing multiple new molecules being evaluated worldwide in more than 300 clinical trials and more than 20 different tumor types.
About AbbVie
AbbVie’s mission is to discover and deliver innovative medicines that solve serious health issues today and address the medical challenges of tomorrow. We strive to have a remarkable impact on people’s lives across several key therapeutic areas: immunology, oncology, neuroscience, eye care, virology, women’s health and gastroenterology, in addition to products and services across its Allergan Aesthetics portfolio. For more information about AbbVie, please visit us at www.abbvie.ca. Follow @abbviecanada on Twitter or find us on LinkedIn.
|
_____________________________________ |
|
|
1 |
Venclexta Product Monograph. AbbVie Corporation, Canada. https://www.abbvie.ca/content/dam/abbvie-dotcom/ca/en/documents/products/VENCLEXTA_PM_EN.pdf. Accessed November 9, 2021 |
|
2 |
Régie de l’assurance maladie du Québec’s Listes des médicaments. https://www.ramq.gouv.qc.ca/fr/a-propos/liste-medicaments-fournis-etablissement. Accessed November 9, 2021 |
|
3 |
Institut national d’excellence en santé et en services sociaux. https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Novembre_2020/Venclexta_2020_10.pdf. Accessed November 9, 2021 |
|
4 |
Lymphoma Canada. Chronic lymphocytic leukemia.www.lymphoma.ca/lymphoma/lymphoma-101/types-lymphoma/cll. Accessed November 9, 2021. |
|
5 |
Canadian Cancer Statistics. Chronic lymphocytic leukemia statistics.www.cancer.ca/en/cancer-information/cancer-type/leukemia-chronic-lymphocytic-cll/statistics/?region=on. Accessed November 9, 2021. |
SOURCE AbbVie Canada
 For further information: Muriel Haraoui, AbbVie Canada, 514-717-3764, muriel.haraoui@abbvie.com
For further information: Muriel Haraoui, AbbVie Canada, 514-717-3764, muriel.haraoui@abbvie.com