Novamind Reports Fiscal Q1 Financial Results and Operating Highlights
- Multistate expansion underway, signed LOI to acquire Arizona-based mental health practice
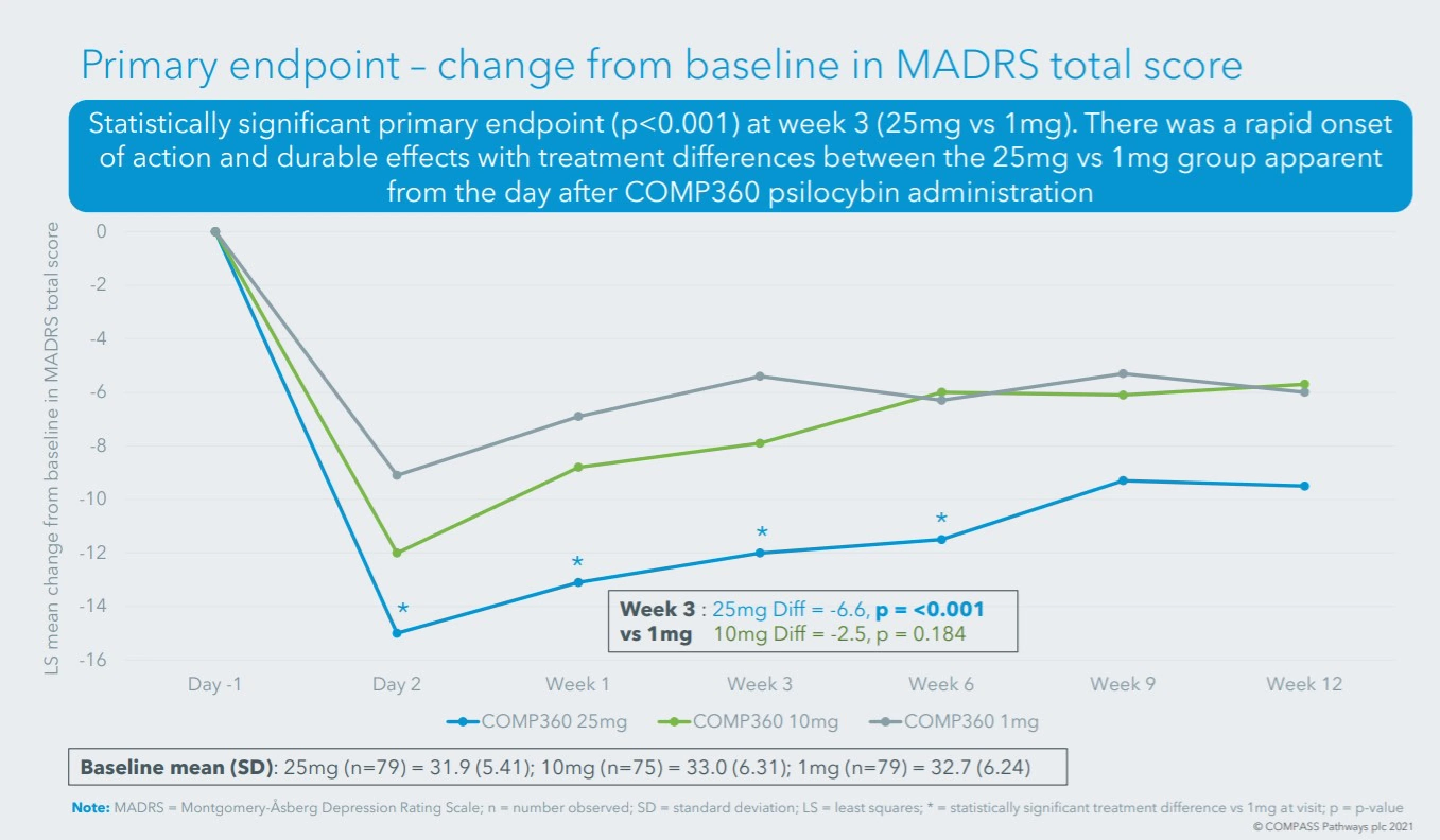
- Total revenue of $1,857,750, +113% when compared to same period last year
- Total working capital of $6,834,011 to fund operations
TORONTO, ON / November 29, 2021 / Novamind Inc. (CSE: NM | OTCQB: NVMDF | FSE: HN2) (“Novamind” or the “Company”), a leading mental health company specialized in psychedelic medicine, reported its fiscal first quarter results for the three months ended September 30, 2021 (“Fiscal Q1 2022”). All results are reported under International Financial Reporting Standards and in Canadian dollars, unless otherwise specified.
“Q1 was a strong start to fiscal year 2022, driven by the addition of another clinic to our network and increased patient demand for our comprehensive range of innovative mental health treatments,” said Yaron Conforti, Novamind’s CEO and Director. “We continue to make progress on our national clinic expansion, most recently with a letter of intent to acquire two locations in Arizona. Our clinical research business has been actively building an exciting pipeline of contracts with leading drug developers.”
Fiscal Q1 2022 Operating Results and Subsequent Events
- Signed letter of intent (“LOI”) to acquire Arizona-based mental health practice, Foundations for Change, the first announcement from a pipeline of accretive transactions
- Leased new clinic to meet patient demand in Utah, the Wheeler Park Clinic, scheduled to open in early 2022
- Opened a new clinic and third research site in Murray, Utah, featuring a new specialized care and research program, Psychedelic Palliative Care by Novamind
- Secured insurance coverage for direct billing of intravenous ketamine for treatment-resistant depression from four major health insurance providers: Blue Cross Blue Shield, the University of Utah, PEHP Health & Benefits and MBA Benefit Administrators
- Unveiled new Company logo and announced rebrand of all subsidiary clinics and research sites
Fiscal Q1 2022 Financial Highlights
- Total revenue of $1,857,750, +113% when compared to same period last year
- Debt-free balance sheet with $5,969,673 in cash and $2,018,971 in marketable securities
- Total working capital of $6,834,011 to fund operations
The following table presents selected financial information from Novamind’s reviewed condensed interim financial statements for the three months ended September 30, 2021. The following information should be read in conjunction with the financial statements and management’s discussion and analysis, which are available under Novamind’s SEDAR profile at www.sedar.com.

About Novamind
Novamind is a leading mental health company enabling safe access to psychedelic medicine through a network of clinics and clinical research sites. Novamind provides ketamine-assisted psychotherapy and other novel treatments through its network of integrative mental health clinics and operates a full-service contract research organization specialized in clinical trials and evidence-based research for psychedelic medicine. For more information on how Novamind is enhancing mental wellness and guiding people through their entire healing journey, visit novamind.ca.
Contact Information
Novamind
Yaron Conforti, CEO and Director
Telephone: +1 (647) 953 9512
Samantha DeLenardo, VP, Communications
Email: media@novamind.ca
Bill Mitoulas, Investor Relations
Email: bill@novamind.ca
Forward-Looking Statements
This news release contains forward-looking statements. All statements other than statements of historical fact included in this release are forward-looking statements that involve risks and uncertainties. There can be no assurance that such statements will prove to be accurate and actual results and future events could differ materially from those anticipated in such statements. Important factors that could cause actual results to differ materially from the Company’s expectations including the risks detailed from time to time in the Company’s public disclosure. The reader is cautioned not to place undue reliance on any forward-looking information. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. The forward-looking statements contained in this news release are made as of the date of this news release and the Company will update or revise publicly any of the included forward-looking statements as expressly required by applicable laws.