PharmaTher Announces Positive Results from Study For Ketamine Microneedle Patch
Successfully delivered ketamine and KETABET™ (ketamine and betaine) via microneedle patch, unlocking the potential for desired dosage forms and pharmacokinetic profiles
Pursuing Phase 2 clinical studies in treatment-resistant depression and pain indications in Q4-2022
TORONTO, June 29, 2022 — PharmaTher Holdings Ltd. (the “Company” or “PharmaTher”) (OTCQB: PHRRF) (CSE: PHRM), a leader in specialty ketamine pharmaceuticals, is pleased to announce that it has successfully completed its research study evaluating the Company’s patented hydrogel-forming microneedle patch, PHARMAPATCH™, to deliver ketamine and KETABET™ (ketamine and betaine anhydrous), which aims to prevent the potential side effects of repeated ketamine treatment for depression and other indications, including suicidal ideation, substance abuse, post-traumatic stress disorder, neurological disorders, and chronic pain.
PHARMAPATCH™ has been shown to successfully deliver esketamine, the S(+) enantiomer of ketamine, which may overcome the drawbacks associated with ketamine administration in an intravenous or nasal spray format. Research results were published in a paper titled “Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery”1.
The aim of the research program led by Prof Ryan Donnelly at Queens University Belfast (“QUB”) was to develop and characterize PHARMAPATCH™ for the transdermal delivery of ketamine and KETABET™ in a sustained manner and finalize production scale-up processes for clinical studies. Characterization of drug recovery and stability before drug permeation from films via hydrogel-forming microneedle array patches (“MAP”) was assessed in vitro using the Franz cell apparatus. Based on the findings from in vitro permeation investigations, lead candidate MAP formulations were selected and brought forward for in vivo testing using Sprague-Dawley rats to assess the ability to achieve sustained plasma concentrations of ketamine and betaine within the therapeutic range for potential antidepressant therapy over the course of 48 hours.
PHARMAPATCH™ is an alternative platform that allows ketamine to be delivered transdermally in a sustained and de-risked manner. The results from this study represent the first time that multiple formulations of PHARMAPATCH™ for the delivery of ketamine and betaine have been developed, characterized and tested in an animal model. Extensive characterisation of each drug-containing polymeric film and hydrogel-forming MAP combination in terms of swelling capacity, insertion capabilities, drug recovery, stability, and ultimately in vitro drug permeation using the Franz cell apparatus allowed the selection of the most promising candidate formulations for in vivo testing. Hydrogel-forming MAP-mediated delivery of ketamine and betaine were compared with intramuscular injection and orally administered solution, respectively. At regular time intervals during the 48-hour rat study, blood sample results demonstrated that PHARMAPATCH™ was able to deliver plasma levels of ketamine (between 70-200 ng/mL) in a controlled manner throughout the study.
The findings of this work support PHARMAPATCH™ as a promising drug delivery platform through which effective, de-risked, and safe ketamine therapies can be delivered. Considering the high degree of flexibility possessed by this delivery system in terms of formulation, the potential for effective treatment regimens extending beyond 48 hours could be developed through further alterations in surface area, application time, and drug loading of these polymeric patches.
The Company is preparing for a planned Phase 2 clinical study to allow for ketamine and KETABET™ microneedle patch evaluation in treatment-resistant depression and chronic pain under the FDA 505(b)(2) regulatory pathway. The Company is manufacturing its ketamine microneedle patches at LTS LOHMANN Therapie-Systeme AG for upcoming GLP pre-clinical pig studies to demonstrate the delivery, dosing and safety profile of ketamine in a comparable animal model to support regulatory filings to conduct human clinical studies in Q4-2022. In addition, scale up of the manufacturing process to support GMP and commercial production is currently underway.
About PHARMAPATCH™
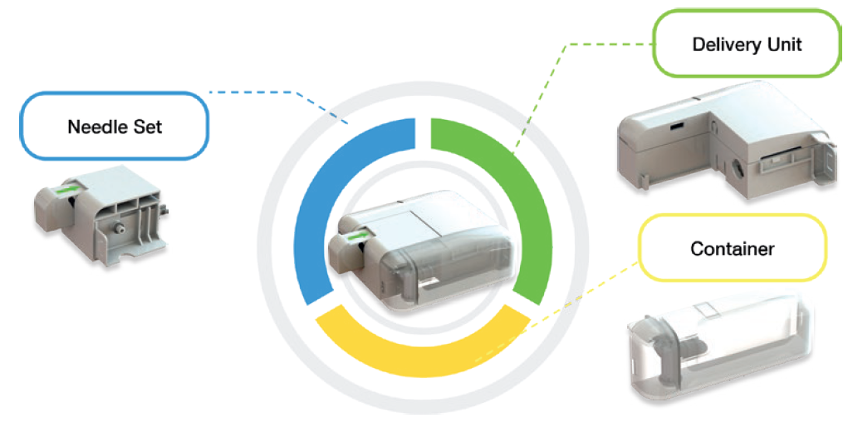
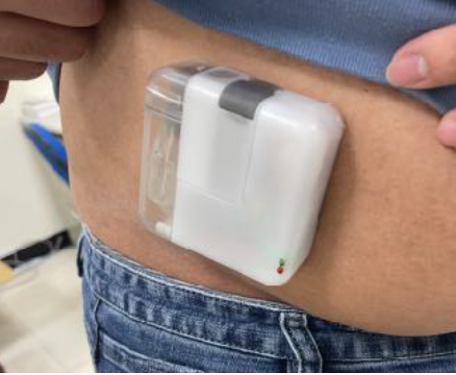
Microneedle-enhanced intradermal delivery is an elegant, efficient and painless method for increasing the skin permeation of many drugs, including ketamine. Transdermal delivery systems offer several advantages over inhalation and intravenous administration. PharmaTher‘s approach with ketamine consists of a 2-part system comprised of a drug-loaded reservoir placed on top of the hydrogel microneedle array. After administration into the skin (i.e. intradermal delivery), the microneedles become hydrated and swell, creating pores for the reservoir to release the drug content into the tissue over the treatment course. Upon removal, the needles are intact, yet rounded, and do not need to be disposed of as sharps. The drug enters the systemic circulation circumventing absorption and first-pass barriers typical for oral delivery. Studies have shown that systemic drug concentrations are reached minutes after administration and maintained over multiple days with transdermal delivery. This system addresses a major unmet need by offering greater ease of administration and including patients with pre-existing conditions that exempt them from oral or inhalation dosing. In addition, it avoids syringe needles, eliminating pain and patient visits to a clinician.
Potential of the Ketamine Microneedle Patch
The Company’s patented hydrogel-forming microneedle (“MN”) patch aims to deliver ketamine for intradermal administration to treat various mental health, neurological and pain disorders. The MN patch consists of hydrogel-forming microneedle arrays and an accompanying reservoir that will overcome limitations by the quantity of drug loaded into the needles or onto the needle surfaces. As such, the MN patch can significantly increase the amount of drug that can permeate through the microneedle array and into the skin.2 The MN patch is tailored for ketamine due to the required drug volume to maximize their therapeutic utility and increase potential market opportunities.
The ketamine MN patch aims to empower patients to dose their medication remotely, safely and conveniently rather than being supervised by a healthcare provider at a certified medical office. The ketamine MN patch has the potential for enabling continuous delivery of ketamine (without pain) with minimal formulation manipulation into systemic circulation while maintaining constant plasma levels for more than 24 hours, which will improve efficacy and compliance for patients.1-2 Also, the ketamine MN patch will incorporate anti-tampering and anti-abuse features that parallel the approach used by commercially available tamper-resistant transdermal fentanyl patches.
About PharmaTher Holdings Ltd.
PharmaTher Holdings Ltd. (OTCQB: PHRRF) (CSE: PHRM) is focused on the development and commercialization of specialty ketamine pharmaceuticals for mental health, neurological, and pain disorders. Learn more at PharmaTher.com.
For more information about PharmaTher, please contact:
Fabio Chianelli
Chief Executive Officer
PharmaTher Holdings Ltd.
Tel: 1-888-846-3171
Email: info@pharmather.com
Website: www.pharmather.com
Neither the Canadian Securities Exchange nor its Regulation Services Provider have reviewed or accept responsibility for the adequacy or accuracy of this release.
Cautionary Statement
This press release contains ‘forward-looking information’ within the meaning of applicable Canadian securities legislation. These statements relate to future events or future performance. The use of any of the words “could”, “intend”, “expect”, “believe”, “will”, “projected”, “estimated”, “potential”, “aim”, “may” and similar expressions and statements relating to matters that are not historical facts are intended to identify forward-looking information and are based on PharmaTher Holdings Ltd. (the “Company”) current belief or assumptions as to the outcome and timing of such future events. Forward-looking information is based on reasonable assumptions that have been made by the Company at the date of the information and is subject to known and unknown risks, uncertainties, and other factors that may cause actual results or events to differ materially from those anticipated in the forward-looking information. Given these risks, uncertainties and assumptions, you should not unduly rely on these forward-looking statements. The forward-looking information contained in this press release is made as of the date hereof, and Company is not obligated to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as required by applicable securities laws. The foregoing statements expressly qualify any forward-looking information contained herein. Factors that could cause actual results to differ materially from those anticipated in these forward-looking statements are described under the caption “Risk Factors” in Company’s management’s discussion and analysis for the three and nine month periods ended February 28, 2022 and 2021 (“MD&A”), dated April 25, 2022, which is available on the Company’s profile at www.sedar.com.
This news release does not constitute an offer to sell or the solicitation of an offer to buy, and shall not constitute an offer, solicitation or sale in any state, province, territory or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state, province, territory or jurisdiction.
Sources:
1.Courtenay, et al. Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery, Journal of Controlled Release, Volume 322, 2020, Pages 177-186.