atai Life Sciences Reports Third Quarter 2021 Financial Results and Corporate Update
-Positive topline results from COMP360’s Phase 2b study-
-RL-007 Phase 2a topline data in cognitive impairment associated with schizophrenia expected end of Q4-
-Continued progress across 11 therapeutic programs, including initiation of Perception’s Phase 2a and DemeRx’s Phase 1/2 trials as well as interim Phase 2a RL-007 readout of first cohort-
-Launch of atai Impact, a philanthropic program to harness the power of innovative mental health approaches for social change-
-Company to host a webcast and conference call today at 08:30am EST-
BERLIN and NEW YORK, Nov. 15, 2021 (GLOBE NEWSWIRE) — atai Life Sciences N.V. (Nasdaq: ATAI) (“atai”), a clinical-stage biopharmaceutical company aiming to transform the treatment of mental health disorders, today reported its financial results for the third quarter ended September 30, 2021, and provided its corporate update.
“Following our IPO in June, we continue to see positive momentum. We have 11 therapeutic programs underway and each clinical development milestone marks progress towards achieving our vision to heal mental health disorders so that everyone, everywhere can live a more fulfilled life,” said Florian Brand, CEO and Co-Founder.
“In response to the heterogeneity of the mental health patient population, we are developing a pharmacologically diverse array of treatments. We intend to support these treatments with innovative digital therapeutics and robust insights from our multi-modal data approach. Our ultimate goal is to tailor our treatments to individual patient needs by using a diverse set of biomarkers. We anticipate further growth of our drug development pipeline and our enabling technologies through our ‘buy and build’ approach and will remain highly active in business development.
“We recently initiated two new clinical trials: a Phase 2a trial with PCN-101 (R-ketamine) for treatment-resistant depression and a Phase 1/2 trial with DMX-1002 (ibogaine) in opioid use disorder. In terms of clinical readouts, just last week we saw positive Phase 2b data from COMPASS Pathways, the first company we funded to rigorously research the potential of psychedelics. This clinical trial demonstrated the rapid onset of effect, large effect size, and durability of COMP360 (a proprietary synthetic formulation of psilocybin) in treatment-resistant depression. We expect another important clinical readout before the end of the year for RL-007 in cognitive impairment associated with schizophrenia.
“The narrow focus on mental health treatments means that every single trial result offers important insights not only for the program in question but for all our programs, allowing us to accelerate the development of novel treatments for patients in need.”
Program Updates
COMP360 (psilocybin):
Program Details: COMP360 is a proprietary formulation of synthetic psilocybin, a 5-HT2A-R agonist being developed as an oral, potentially rapid-acting antidepressant.
Recent Advancements:
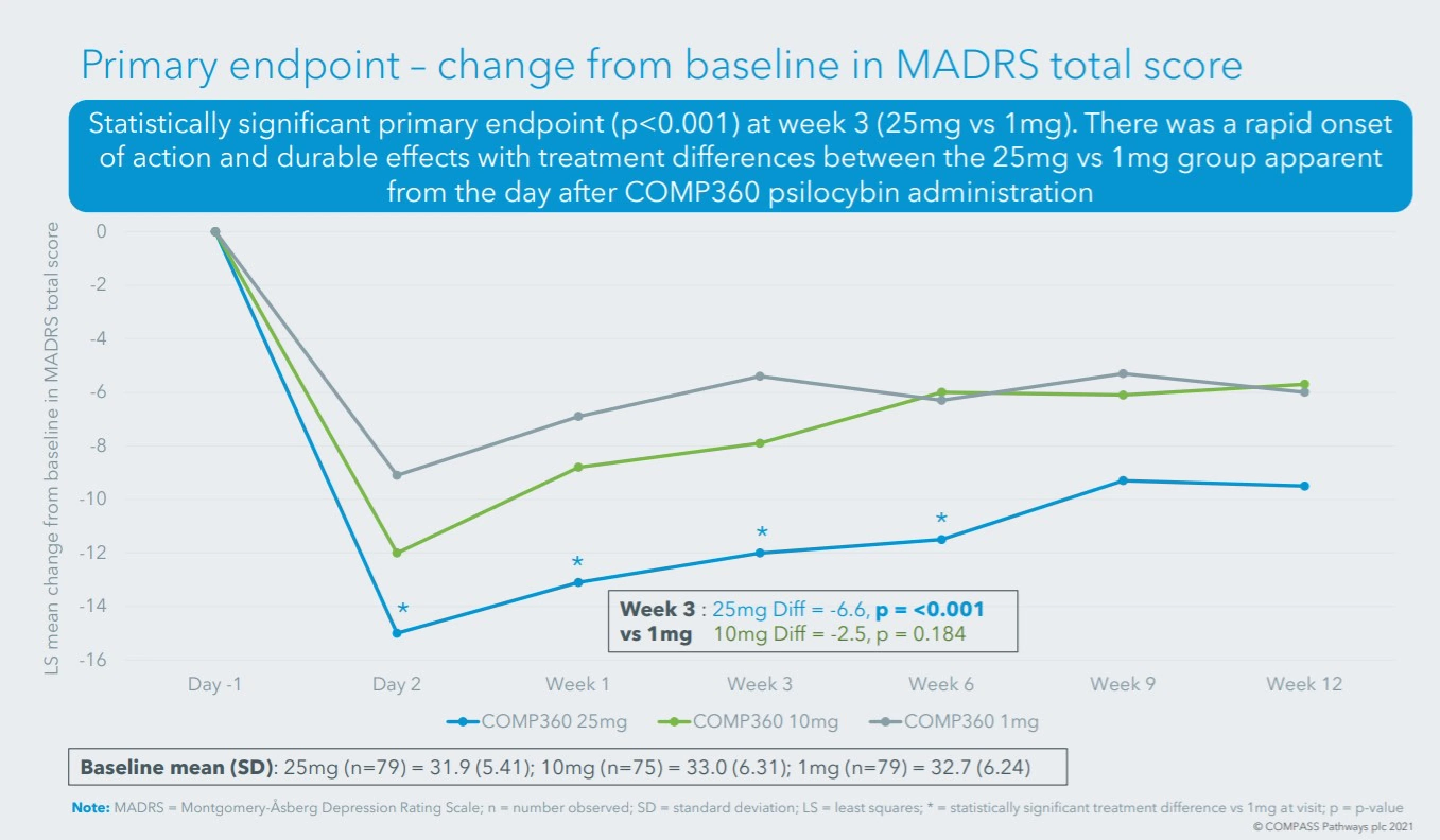
- In November, COMPASS announced positive topline results from its Phase 2b randomized, controlled, double-blind, dose-controlled trial of COMP360 psilocybin therapy for treatment-resistant depression.
- The 233-patient study met its primary endpoint, showing a 6.6-point reduction on the Montgomery-Åsberg Depression Rating Scale (MADRS) total score from baseline to 3 weeks when comparing the 25mg dose to the 1mg dose.
- COMP360 also showed both rapid response and durability of efficacy. Rapid onset of action with statistically significant treatment differences between the 25mg vs 1mg groups were apparent the day after COMP360 psilocybin administration. Responder rates at week 12 based upon a ≥50% decrease in MADRS total score from baseline were 32.9% and 16.4% for psilocybin doses of 25mg and 1mg respectively. Remitter rates at week 12 based upon a MADRS total score ≤10 were 26.6% and 11.4% for psilocybin doses of 25mg and 1mg respectively.
- COMP360 was generally well tolerated.
Upcoming Milestones:
- A pivotal Phase 3 study is anticipated to launch in 2022.
Affiliate: COMPASS Pathways
PCN-101 (R-ketamine):
Program Details: PCN-101 is a parenteral formulation of R-ketamine, a glutamatergic modulator being developed as a potentially rapid-acting antidepressant, with the potential for at-home treatment.
Recent Advancements:
- In September 2021, Perception Neuroscience initiated the Phase 2 trial of PCN-101 (R-ketamine) for TRD. This randomized, double blind, placebo-controlled trial is designed to assess the efficacy, safety, dose response and duration of action in patients with TRD.
Upcoming Milestones:
- Topline data are expected at the end of 2022.
Affiliate: Perception Neuroscience
RL-007:
Program Details: RL-007, a cholinergic, glutamatergic and GABA-B receptor modulator, is an orally available compound that is being developed for the treatment of cognitive impairments associated with schizophrenia (CIAS). The currently active open-label, multi-dose, biomarker-focused Phase 2a trial of RL-007 in patients with CIAS is designed to evaluate the compound’s safety, tolerability and its impact on electroencephalogram-based biomarkers.
Recent Advancements:
- Following the encouraging results of a recently completed interim analysis of Quantitative Electroencephalogram (qEEG) data from the eight patients in the first cohort, atai advanced a portion of a future milestone payment aiming to accelerate initiation of the subsequent trial, which, broadly, will be a double-blind, placebo controlled, proof-of-concept study focused on more traditional cognitive endpoints, including subsets of the MATRICS battery.
Upcoming Milestones:
- Topline data are expected by the end of 2021.
Affiliate: Recognify Life Sciences
GRX-917 (deuterated etifoxine):
Program Details: GRX-917 is an oral formulation of a deuterated version of etifoxine, a mitochondrial translocator protein agonist, designed to provide potentially rapid anxiolytic activity with improved tolerability compared to current treatments for anxiety in the United States.
Recent Advancements:
- The Phase 1 randomized, double blind, placebo-controlled trial of GRX-917 is designed to evaluate the compound’s safety, tolerability, pharmacokinetics, as well as pharmacodynamics using qEEG. We have recently completed the single ascending dose (SAD) component of the trial, and dosing in the multiple ascending dose (MAD) component is ongoing.
Upcoming Milestones:
- Topline data are expected in mid-2022.
Affiliate: GABA Therapeutics
DMX-1002 (ibogaine):
Program Details: DMX-1002 is an oral formulation of ibogaine, a cholinergic, glutamatergic and monoaminergic receptor modulator being developed for the treatment of opioid use disorder (OUD).
Recent Advancements:
- In September 2021, DemeRx IB dosed the first subject in a Phase 1/2a trial of DMX-1002. This trial is designed to assess the safety, tolerability, pharmacokinetics, and efficacy of DMX-1002 and will inform future studies in patients with OUD.
Upcoming Milestones
- Topline safety data are expected in early 2022.
Affiliate: DemeRx IB
Q3 and Recent Corporate Updates
Discovery Programs
- Launched PsyProtix, a precision psychiatry company creating new chemical entities targeting mitochondrial dysfunction related to TRD and other mental health conditions. PsyProtix is currently engaged in discovery and preclinical development and expects to launch clinical trials in 2023.
- Created and pharmacologically tested over 250 novel compounds at EntheogeniX based upon structures generated using computational chemistry approach. Lead candidate selection is currently ongoing.
Formulation Technologies
- Entered into an expanded agreement with strategic partner IntelGenx, building on positive early feasibility data, to support IntelGenx’s graduation from the TSX Venture Exchange to the Toronto Stock Exchange. As part of the strategic partnership, IntelGenx has exclusively partnered with atai to develop formulations of compounds for the prevention or treatment of mental health disorders.
- Completed a proof-of-principle study demonstrating that InnarisBio’s sol-gel based excipient technology can effectively transport compounds from the nose directly to the brain in an animal model. InnarisBio is developing its nose-to-brain excipient technology to facilitate potentially rapid, non-invasive entry into the brain for use across various drug candidates in atai’s pipeline, including Revixia, Neuronasal, and DemeRx.
Digital Therapeutics
- Initiated a user acceptability testing of our digital therapeutics (DTx) app, one of our key enabling technologies, in patients with TRD receiving ketamine treatment.
- In addition, we have assembled a proof-of-concept, EEG- and VR-based digital therapeutic device to support patients undergoing psychedelic therapy, and we have kicked off user feedback testing to optimize across product parameters.
- Both technologies are expected to be implemented in Viridia and Revixia Phase 1 trials and DemeRx IB Phase 2 trial starting next year.
New Initiatives
- Launched atai Impact, atai’s philanthropic program, established to support and collaborate with nonprofits and institutions that share atai Life Sciences’ vision, with the key pillars of: advancing education, expanding access, and supporting the wider ecosystem of mental health care. atai Impact has an initial focus on the psychedelics sector, given its emerging potential in tackling the growing mental health crisis. Its formation is underpinned by atai’s belief that harmonization across commercial and non-profit entities represents the best path forward to harness the power of innovative mental health approaches for positive social change. The atai Impact program will be initially funded by 1% of the gross proceeds from our June 2021 IPO and founders’ and shareholders’ contributions.
Third Quarter 2021 Financial Results
Cash and Cash Equivalents
Cash and cash equivalents totaled $430.3 million as of September 30, 2021, compared to $97.2 million as of December 31, 2020. The nine month increase of $333.1 million is attributed to net proceeds of $231.6 million from atai’s IPO, net proceeds of $166.4 million from Series C and Series D and common stock issuances, $20.0 million of license revenue proceeds, and $10.1 million proceeds from the sale of investments and issuance and conversion of convertible notes. Offsetting were cash payments of $32.6 million for investments in platform companies and other assets, and $62.4 million in net operating expenses and effect of foreign exchange rate changes.
Operating Costs and Expenses
Research and development (R&D) expenses were $13.4 million and $35.0 million for the three and nine months ended September 30, 2021, respectively, as compared to $3.1 million and $8.1 million for the same prior year periods. The increase of $10.3 million and $26.9 million, respectively, were attributable to personnel costs, including stock-based compensation expense, and increased contract research organization expenses related to advancements in atai’s R&D programs.
atai recorded acquisition of in-process R&D expense of $9.0 million for the nine months ended September 30, 2021, relating to its investments in Neuronasal and InnarisBio.
General and administrative expenses for the three and nine months ended September 30, 2021 were $20.3 million and $66.9 million, respectively, as compared to $4.3 million and $8.7 million in the same prior year periods. The increases of $16.0 million and $58.2 million, respectively, were attributable to personnel costs, including stock-based compensation expense, professional fees, and other costs related to support of atai’s platform growth and public company requirements.
Total stock-based compensation expense for the three and nine months ended September 30, 2021 was $12.2 million and $50.0 million, respectively, as compared to $2.1 and $2.2 for the comparable prior year periods, reflecting the recognition of expense related to the achievement of IPO performance-based partial vesting conditions.
Net loss attributable to atai shareholders for the three and nine months ended September 30, 2021 was $31.2 million and $78.9 million, respectively, as compared to $83.2 million and $83.2 million for the comparable prior year periods.
Conference Call Information
atai will host a conference call and live audio webcast today at 08:30am EST to discuss its financial results and provide a corporate update. To access the live conference call, please dial 877-407-3982 from the United States, or +1 (201) 493-6780 internationally, using the conference ID: 13724750. The live and archived webcast of this call will be available in the “Events” section of the atai Life Sciences website at ir.atai.life. An archived copy of the webcast will be available on the atai website for at least 30 days after the conference call.
About atai Life Sciences
atai is a clinical-stage biopharmaceutical company aiming to transform the treatment of mental health disorders. atai was founded in 2018 as a response to the significant unmet need and lack of innovation in the mental health treatment landscape. atai is dedicated to acquiring, incubating and efficiently developing innovative therapeutics to treat depression, anxiety, addiction, and other mental health disorders.
atai’s business model combines funding, technology, scientific and regulatory expertise with a focus on psychedelic therapy and other drugs with differentiated safety profiles and therapeutic potential. By pooling resources and best practices, atai aims to responsibly accelerate the development of new medicines across its companies, seeking to effectively treat and ultimately heal mental health disorders.
atai’s mission is to bridge the gap between what the mental healthcare system currently provides and what patients need. atai is headquartered in Berlin, with offices in New York and London. For more information, please visit www.atai.life.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “anticipate,” “initiate,” “could,” “would,” “project,” “plan,” “potentially,” “preliminary,” “likely,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements include express or implied statements relating to, among other things, our future operating results and financial position; the success, cost and timing of development of our product candidates, including the progress of preclinical and clinical trials and related milestones; the commercialization of our current product candidates and any other product candidates we may identify and pursue, if approved, including our ability to successfully build a specialty sales force and commercial infrastructure to market our current product candidates and any other product candidates we may identify and pursue; the timing of and our ability to obtain and maintain regulatory approvals; our business strategy and plans; potential acquisitions; and the plans and objectives of management for future operations and capital expenditures. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond our control and which could cause actual results, levels of activity, performance or achievements to differ materially from those expressed or implied by these forward-looking statements.
We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including without limitation: we are a clinical-stage biopharmaceutical company and have incurred significant losses since our inception, and we anticipate that we will continue to incur significant losses for the foreseeable future; we will require substantial additional funding to achieve our business goals, and if we are unable to obtain this funding when needed and on acceptable terms, we could be forced to delay, limit or terminate our product development efforts; our limited operating history may make it difficult to evaluate the success of our business and to assess our future viability; we have never generated revenue and may never be profitable; our product candidates contain controlled substances, the use of which may generate public controversy; clinical and preclinical development is uncertain, and our preclinical programs may experience delays or may never advance to clinical trials; we rely on third parties to assist in conducting our clinical trials and some aspects of our research and preclinical testing, and those clinical trials, including progress and related milestones, may be impacted by several factors including the failure by such third parties to meet deadlines for the completion of such trials, research, or testing, changes to trial sites and other circumstances; we currently rely on qualified therapists working at third-party clinical trial sites to administer certain of our product candidates in our clinical trials and we expect this to continue upon approval, if any, of our current or future product candidates; if third-party sites fail to recruit and retain a sufficient number of therapists or effectively manage their therapists, our business, financial condition and results of operations would be materially harmed; we cannot give any assurance that any of our product candidates will receive regulatory approval, which is necessary before they can be commercialized; research and development of drugs targeting the central nervous system, or CNS, is particularly difficult, and it can be difficult to predict and understand why a drug has a positive effect on some patients but not others; we face significant competition in an environment of rapid technological and scientific change; third parties may claim that we are infringing, misappropriating or otherwise violating their intellectual property rights, the outcome of which would be uncertain and may prevent or delay our development and commercialization efforts; a change in our effective place of management may increase our aggregate tax burden; we identified material weaknesses in connection with our internal control over financial reporting; and a pandemic, epidemic, or outbreak of an infectious disease, such as the COVID-19 pandemic, may materially and adversely affect our business, including our preclinical studies, clinical trials, third parties on whom we rely, our supply chain, our ability to raise capital, our ability to conduct regular business and our financial results. Other risk factors include the important factors described in the section titled “Risk Factors” in our final prospectus, dated June 17, 2021, filed with the Securities and Exchange Commission (“SEC”) pursuant to Rule 424(b) under the Securities Act, and in our other filings with the SEC, that may cause our actual results, performance or achievements to differ materially and adversely from those expressed or implied by the forward-looking statements.
Any forward-looking statements made herein speak only as of the date of this press release, and you should not rely on forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, performance, or achievements reflected in the forward-looking statements will be achieved or will occur. Except as required by applicable law, we undertake no obligation to update any of these forward-looking statements for any reason after the date of this press release or to conform these statements to actual results or revised expectations.
Contact Information
Media Contact:
Camilla Dormer
VP, Communications, atai Life Sciences
Email: camilla@atai.life
Investor Contact:
Chad Messer
VP, Investor Relations, atai Life Sciences
Email: chad@atai.life
| ATAI LIFE SCIENCES N.V. | |||||||||||||||||
| CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS | |||||||||||||||||
| (Amounts in thousands, except share and per share amounts) | |||||||||||||||||
| (unaudited) | |||||||||||||||||
| Three Months Ended | Nine Months Ended | ||||||||||||||||
| September 30, | September 30, | ||||||||||||||||
| 2021 | 2020 | 2021 | 2020 | ||||||||||||||
| License revenue | $ | 266 | $ | – | $ | 20,146 | $ | – | |||||||||
| Operating expenses: | |||||||||||||||||
| Research and development | 13,363 | 3,058 | 34,974 | 8,056 | |||||||||||||
| Acquisition of in-process research and development | – | – | 8,934 | 120 | |||||||||||||
| General and administrative | 20,264 | 4,328 | 66,868 | 8,749 | |||||||||||||
| Total operating expenses | 33,627 | 7,386 | 110,776 | 16,925 | |||||||||||||
| Loss from operations | (33,361 | ) | (7,386 | ) | (90,630 | ) | (16,925 | ) | |||||||||
| Other income (expense), net | 6,887 | (13,942 | ) | 2,608 | 6,352 | ||||||||||||
| Net loss before income taxes | (26,474 | ) | (21,328 | ) | (88,022 | ) | (10,573 | ) | |||||||||
| Provision for income taxes | (368 | ) | (4 | ) | (432 | ) | (4 | ) | |||||||||
| Gain on investment dilution | – | – | 16,923 | – | |||||||||||||
| Losses from investments in equity method investees, net of tax | (4,800 | ) | (61,862 | ) | (9,440 | ) | (73,693 | ) | |||||||||
| Net loss | (31,642 | ) | (83,194 | ) | (80,971 | ) | (84,270 | ) | |||||||||
| Net income (loss) attributable to redeemable noncontrolling | |||||||||||||||||
| interests and noncontrolling interests | (484 | ) | 1 | (2,040 | ) | (1,021 | ) | ||||||||||
| Net loss attributable to ATAI Life Sciences N.V. stockholders | $ | (31,158 | ) | $ | (83,195 | ) | $ | (78,931 | ) | $ | (83,249 | ) | |||||
| Net loss per share attributable to ATAI Life Sciences N.V. stockholders– basic and diluted | $ | (0.21 | ) | $ | (0.92 | ) | $ | (0.59 | ) | $ | (0.92 | ) | |||||
| Weighted average common shares outstanding attributable to ATAI Life Sciences N.V. stockholders — basic and diluted |
151,130,212 | 90,709,312 | 134,334,685 | 90,709,312 | |||||||||||||
| ATAI LIFE SCIENCES N.V. | |||||||
| CONDENSED CONSOLIDATED BALANCE SHEET | |||||||
| (Amounts in thousands) | |||||||
| September 30, | December 31, | ||||||
| 2021 | 2020 | ||||||
| (unaudited) | (1) | ||||||
| Assets | |||||||
| Cash and cash equivalents | $ | 430,308 | $ | 97,246 | |||
| Prepaid expenses and other current assets | 11,551 | 2,076 | |||||
| Short term notes receivable – related party | – | 226 | |||||
| Property and equipment, net | 138 | 71 | |||||
| Deferred offering costs | – | 1,575 | |||||
| Equity method investments | 15,086 | – | |||||
| Other investments held at fair value | 6,816 | – | |||||
| Other investments | 14,256 | 8,044 | |||||
| Long term notes receivable | 908 | 911 | |||||
| Long term notes receivable – related parties | 3,784 | 1,060 | |||||
| Other assets | 1,262 | 339 | |||||
| Total assets | $ | 484,109 | $ | 111,548 | |||
| Liabilities and Stockholders’ Equity | |||||||
| Accounts payable | $ | 1,974 | $ | 3,083 | |||
| Accrued liabilities | 13,075 | 9,215 | |||||
| Current portion of contingent consideration liability – related parties | 50 | – | |||||
| Deferred revenue | 180 | – | |||||
| Short-term notes payable | 38 | – | |||||
| Non-current portion of contingent consideration liability – related parties | 1,947 | 1,705 | |||||
| Convertible promissory notes – related parties, net of discounts and deferred issuance costs | 800 | 1,199 | |||||
| Convertible promissory notes and derivative liability | – | 978 | |||||
| Other liabilities | 3,285 | – | |||||
| Total stockholders’ equity attributable to ATAI Life Sciences N.V. stockholders | 453,186 | 90,822 | |||||
| Noncontrolling interests | 9,574 | 4,546 | |||||
| Total liabilities and stockholders’ equity | $ | 484,109 | $ | 111,548 | |||
| (1) The condensed consolidated financial statements as of and for the year ended December 31, 2020 are derived from the audited consolidated financial statements as of that date. | |||||||