Journal of the Royal Society of Medicine Publishes New COVID-19 Suicide Research Led by Braxia Scientific CEO Dr. Roger McIntyre
- Findings Underscore Company Core Objectives to Develop Ketamine Derivatives as Additional Measures to Further Reduce Suicidality
TORONTO, ONTARIO October 21, 2021 – Braxia Scientific Corp. (“Braxia”, or the “Company”), (CSE: BRAX) (OTC: BRAXF) (FWB: 496), a medical research company with clinics providing innovative ketamine treatments for persons with depression and related disorders, is pleased to announce the publication of a new study led by Braxia Scientific CEO Dr. Roger McIntyre in the Journal of the Royal Society of Medicine.
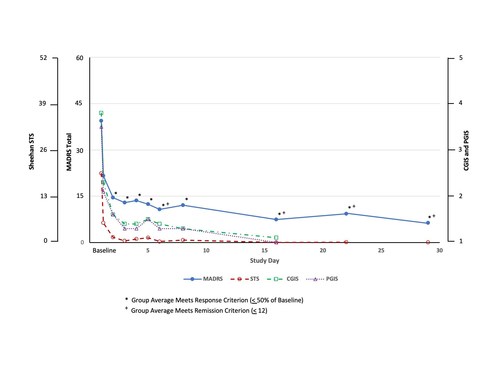
The publication, entitled “Suicide reduction in Canada during the COVID-19 pandemic: lessons informing national prevention strategies for suicide reduction” was initiated to evaluate the impact of federal, public health and social support programs on national suicide rates in Canada, which were put in place to mitigate the abrupt changes to social and financial provisions brought on by the COVID-19 pandemic.
“Reducing the rate of suicide rates is one of Braxia Scientific’s core objectives and should be a national imperative in Canada and globally,” said Dr. McIntyre. “The findings presented in this research should be used to inform public policy and develop strategies for decreasing suicide rates in Canada.”
The overall suicide mortality rate decreased in Canada from 10.82 deaths per 100,000 in March 2019 – February 2020 (i.e., 4,090 suicide deaths in a population of 37,802,043) to 7.34 per 100,000 (i.e., 2,790 deaths in a population of 38,008,005) in March 2020 – February 2021, an absolute difference of 1,300 suicide deaths. Meanwhile, the Canadian unemployment rate had changed from an average monthly rate of 5.7% in 2019 to 9.5% in 2020. Although the data are provisional, they represent a promising trend.
Coinciding with the reduced rate of suicide in Canada, during the time of observation, there was an increase in the availability of virtual psychiatric healthcare, including but not limited to psychotherapy and counselling service.
“In addition to public policies that prioritize psychiatric services generally, especially for persons at risk for suicide, we believe that we can reduce the rate of suicide even further through the innovative Ketamine treatments we are providing today and through new potential derivatives in the future” added Dr. McIntyre.
“With the current and upcoming clinical trials at Braxia Scientific centers, we are working hard to investigate the effects and potential of psychedelics like ketamine and psilocybin as therapies for treatment-resistant depression and other brain-based disorders that are associated with suicide.”
In addition, the results of the study suggest that government interventions that broadly aim to reduce measures of insecurity (such as those relating to finances, housing and health) and provide timely psychiatric services, should be prioritized as part of a national suicide reduction strategy, not only during but after termination of the COVID-19 pandemic.
The full text of the study can be found here.
About Braxia Scientific Corp.
Braxia Scientific is a medical research company with clinics that provide innovative ketamine treatments for persons with depression and related disorders. Through its medical solutions, Braxia aims to reduce the illness burden of brain-based mental disorders such as major depressive disorder among others. Braxia is primarily focused on (i) owning and operating multidisciplinary clinics, providing treatment for mental health disorders, and (ii) research activities related to discovering and commercializing novel drugs and delivery methods. Braxia seeks to develop ketamine and derivatives and other psychedelic products from its IP development platform. Through its wholly owned subsidiary, the Canadian Rapid Treatment Center of Excellence Inc., Braxia currently operates multidisciplinary community-based clinics offering rapid-acting treatments for depression located in Mississauga, Toronto, Ottawa, and Montreal.
ON BEHALF OF THE BOARD
“Dr. Roger S. McIntyre”
Dr. Roger S. McIntyre
FOR FURTHER INFORMATION PLEASE CONTACT:
Braxia Scientific Corp.
Tel: 416-762-2138
Email: info@braxiascientific.com
Website: www.braxiascientific.com
The CSE has not reviewed and does not accept responsibility
for the accuracy or adequacy of this release.
Forward-looking Information Cautionary Statement
This news release contains forward-looking statements within the meaning of applicable securities laws. All statements that are not historical facts, future estimates, plans, programs, forecasts, projections, objectives, assumptions, expectations, or beliefs of future performance are “forward-looking statements.”
Forward-looking statements include statements about the intended promise of ketamine-based treatments for depression and the potential for ketamine to treat other emerging psychiatric disorders, such as Bipolar Depression. Such forward- looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results, events, or developments to be materially different from any future results, events or developments expressed or implied by such forward-looking statements. Such risks and uncertainties include, among others, the failure of ketamine, psilocybin and other psychedelics to provide the expected health benefits and unanticipated side effects, dependence on obtaining and maintaining regulatory approvals, including acquiring and renewing federal, provincial, municipal, local or other licenses and engaging in activities that could be later determined to be illegal under domestic or international laws. Ketamine and psilocybin are currently Schedule I and Schedule III controlled substances, respectively, under the Controlled Drugs and Substances Act, S.C. 1996, c. 19 (the “CDSA”) and it is a criminal offence to possess such substances under the CDSA without a prescription or a legal exemption. Health Canada has not approved psilocybin as a drug for any indication, however ketamine is a legally permissible medication for the treatment of certain psychological conditions. It is illegal to possess such substances in Canada without a prescription.
These factors should be considered carefully, and readers are cautioned not to place undue reliance on such forward-looking statements.
Although the Company has attempted to identify important risk factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other risk factors that cause actions, events or results to differ from those anticipated, estimated or intended. Additional information identifying risks and uncertainties that could affect financial results is contained in the Company’s filings with Canadian securities regulators, including the Amended and Restated Listing Statement dated April 15, 2021, which are available at www.sedar.com. There can be no assurance that forward-looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in forward-looking statements.