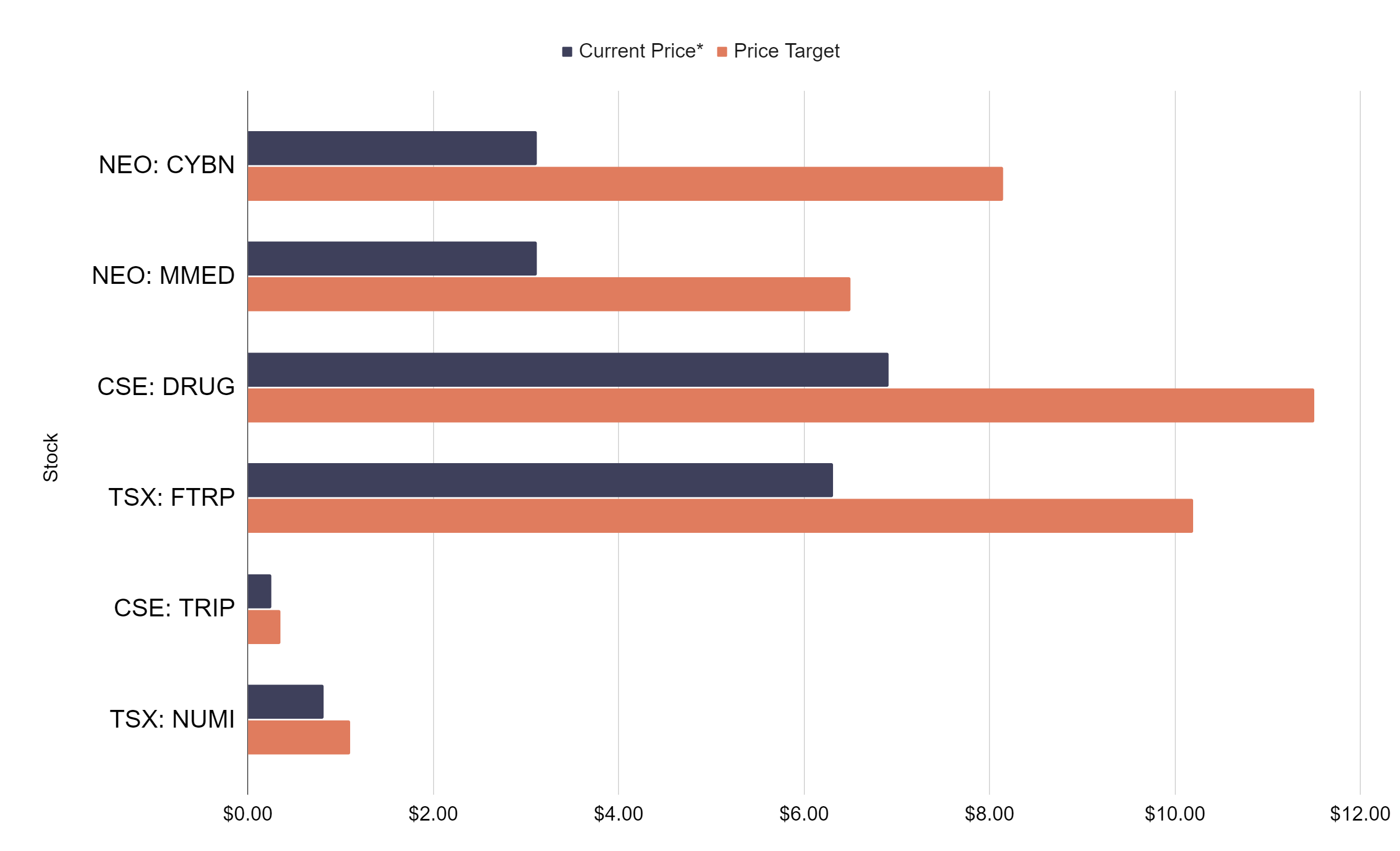
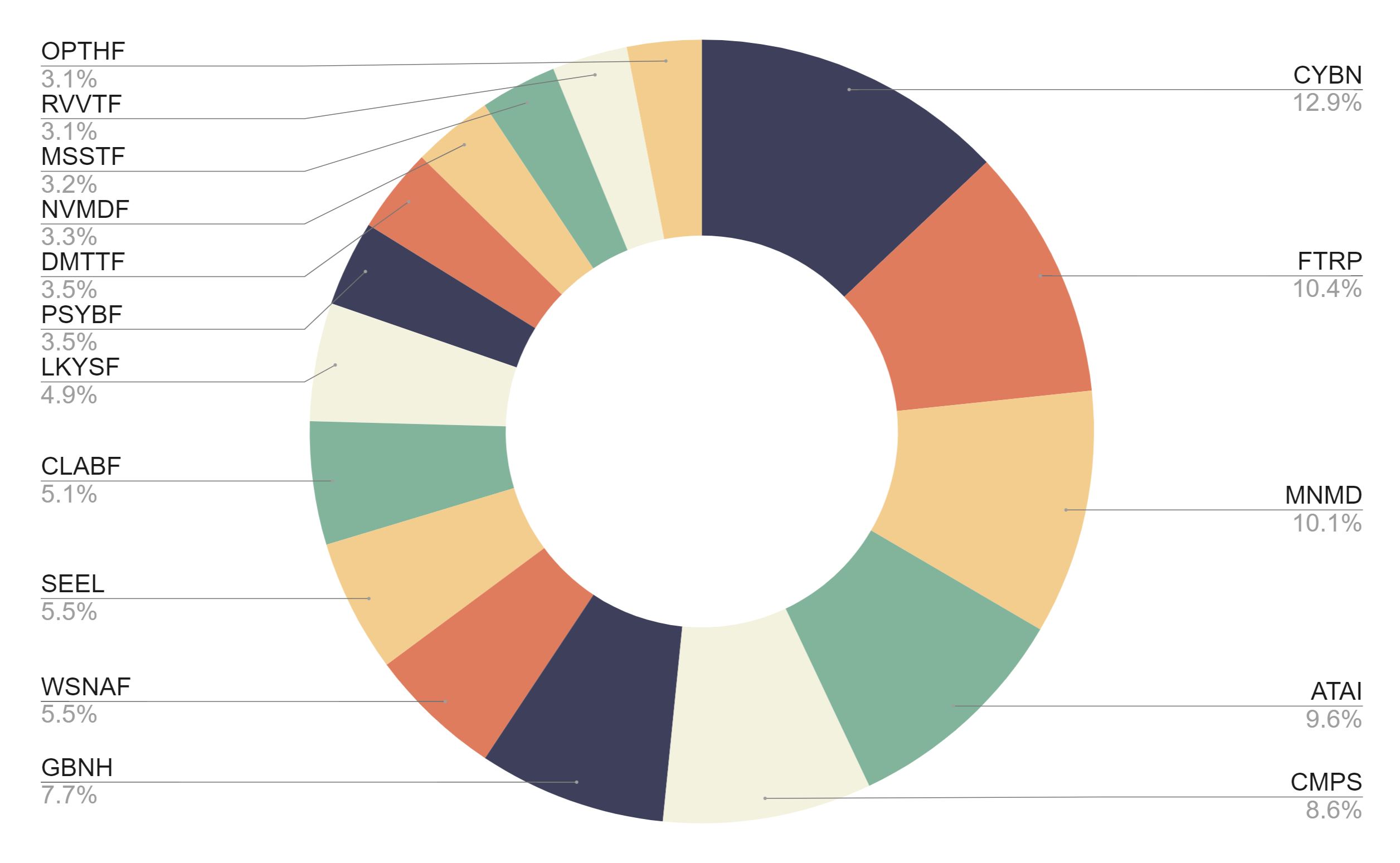
OXFORD, Ohio and COCONUT CREEK, Fla., Sept. 17, 2021 /CNW/ – PsyBio Therapeutics Corp. (TSXV: PSYB) (OTCQB: PSYBF) (“PsyBio” or the “Company“), an intellectual property driven biotechnology company developing novel, bespoke, and psycho-targeted therapeutics to potentially improve mental and neurological health, today announced that it has been included in AdvisorShares Psychedelics Exchange Traded Fund (the “ETF“).
The ETF will actively manage investments in the emerging psychedelic drugs sector, offering exposure to those biotechnology, pharmaceutical and life sciences companies that the ETF sees as leading the industry. The ETF currently includes 16 psychedelics companies and is listed on the NYSE Arca under the ticker symbol (NYSE: PSIL).
Evan Levine, Chief Executive Officer and Chairman of PsyBio, said, “The inclusion of PsyBio in AdvisorShares Psychedelics ETF recognizes us as a leader in the psychedelics industry, as we continue to research and develop innovative treatments to potentially improve mental and neurological health. We want to thank AdvisorShares for this recognition, which is a testament to the work the Company is advancing in this area. We are proud to be included in the ETF and look forward to continuing engagement with investors on the development of PsyBio’s therapeutics program to treat specific clinical conditions.”
Additional information on the AdvisorShares Psychedelics ETF can be found here.
About PsyBio Therapeutics Corp.
PsyBio Therapeutics is an intellectual property driven biotechnology company developing novel, bespoke, psycho-targeted therapeutics to potentially improve mental and neurological health. The team has extensive experience in drug discovery based on synthetic biology and metabolic engineering as well as clinical and regulatory expertise progressing drugs through human studies and regulatory protocols. Research and development is currently ongoing for naturally occurring psychoactive tryptamines originally discovered in different varieties of hallucinogenic mushrooms, other tryptamines and phenethylamines and combinations thereof. The Company utilizes a Bio Medicinal Chemistry approach to therapeutic development, in which psychoactive compounds can be utilized as a template upon which to develop precursors and analogs, both naturally and non-naturally occurring.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that constitute “forward-looking information” (“forward-looking information“) within the meaning of applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking information and are based on expectations, estimates and projections as at the date of this news release. Any statement that discusses predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as “expects”, or “does not expect”, “is expected”, “anticipates” or “does not anticipate”, “plans”, “budget”, “scheduled”, “forecasts”, “estimates”, “believes” or “intends” or variations of such words and phrases or stating that certain actions, events or results “may” or “could”, “would”, “might” or “will” be taken to occur or be achieved) are not statements of historical fact and may be forward-looking information. Forward looking-statements in this press release include, amongst other things, statements regarding the ability of PsyBio to develop novel formulations to potentially treat neurologic and psychologic conditions and other disorders.
In disclosing the forward-looking information contained in this press release, the Company has made certain assumptions, including that: PsyBio will be successful in protecting its intellectual property; PsyBio will be successful in discovering new valuable target molecules; PsyBio will file its initial pre-Investigational New Drug (“IND“) Application request and IND Application within anticipated timeframes; PsyBio will be successful in obtaining IND Applications and will be able to obtain all necessary approvals for clinical trials; PsyBio will be successful in launching clinical trials; the results of preclinical safety and efficacy testing will be favourable; PsyBio’s technology will be safe and effective; a confirmed signal will be identified in PsyBio’s selected indications; and that drug development involves long lead times, is very expensive and involves many variables of uncertainty. Although the Company believes that the expectations reflected in such forward-looking information are reasonable, it can give no assurance that the expectations of any forward-looking information will prove to be correct. Known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking information. Such factors include, but are not limited to: compliance with extensive government regulations; domestic and foreign laws and regulations adversely affecting PsyBio’s business and results of operations; decreases in the prevailing process for psilocybin and nutraceutical products in the markets in which PsyBio operates; the impact of COVID-19; and general business, economic, competitive, political and social uncertainties. Accordingly, readers should not place undue reliance on the forward-looking information contained in this press release. Except as required by law, the Company disclaims any intention and assumes no obligation to update or revise any forward-looking information to reflect actual results, whether as a result of new information, future events, changes in assumptions, changes in factors affecting such forward-looking information or otherwise.
PsyBio makes no medical, treatment or health benefit claims about PsyBio’s proposed products. The United States Food and Drug Administration (“FDA“) or other similar regulatory authorities have not evaluated claims regarding psilocybin and other next generation psychoactive compounds. The efficacy of such products has not been confirmed by FDA-approved research. There is no assurance that the use of psilocybin and other psychoactive compounds can diagnose, treat, cure, or prevent any disease or condition. Vigorous scientific research and clinical trials are needed. PsyBio has not conducted clinical trials for the use of its intellectual property. Any references to quality, consistency, efficacy and safety of potential products do not imply that PsyBio verified such in clinical trials or that PsyBio will complete such trials. If PsyBio cannot obtain the approvals or research necessary to commercialize its business, it may have a material adverse effect on the PsyBio’s performance and operations.
The TSX Venture Exchange (“TSXV“) has neither approved nor disapproved the contents of this news release. Neither the TSXV nor its Regulation Services Provider (as that term is defined in the policies of the TSXV) accepts responsibility for the adequacy or accuracy of this release.
SOURCE PsyBio Therapeutics Corp.

For further information: Evan Levine, CEO, PsyBio Therapeutics Corp., t: 513.449.9585, e: ir@psybiolife.com; Investor Enquiries: Valter Pinto or Tim Regan, KCSA Strategic Communications, t: 212.896.1254, e: valter@kcsa.com