Better Plant Congratulates Affiliate Neonmind On Recent Achievements
Vancouver, B.C. – November 10, 2021: Better Plant Sciences Inc. (CSE: PLNT) (OTCQB: VEGGF) (FRA: YG3) (“Better Plant” or the “Company”), a wellness company, congratulated its affiliate NeonMind Biosciences Inc. (“NeonMind”) on its recent achievements and advancements toward its drug development program and planned specialty clinics launch.
On November 8, 2021, NeonMind formed an alliance with SRx Health Solutions (“SRx”). SRx is a leading Canadian specialty healthcare services and medical treatment provider, to establish and operate a network of NeonMind-branded specialty clinics to deliver evidence-backed innovative treatments for a variety of mental health needs. NeonMind will leverage SRx’s nationwide network of over 70 clinics, as well as its operational capabilities, to bring NeonMind’s unique treatment protocols to underserved populations in Canada.
Prior to its alliance with SRx, NeonMind was ranked third in Psychedelia Magazine’s “8 Industry Innovators” segment within the magazine’s inaugural Winter 2022 issue. NeonMind was recognized for its dual approach in bringing psychedelic treatments to patients in need through its anticipated specialty mental health clinic launch and its drug development program for novel obesity treatments.
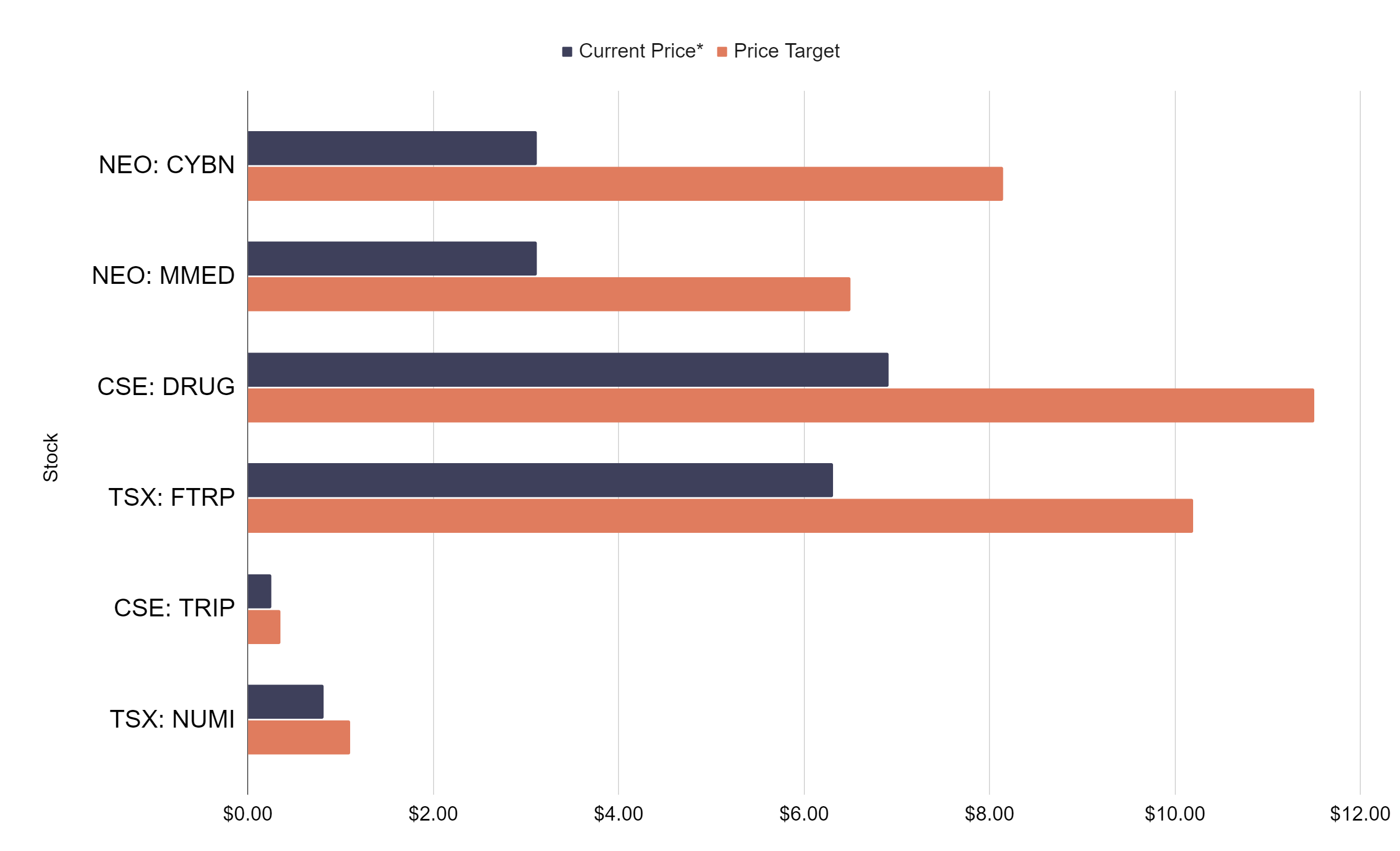
Better Plant congratulates NeonMind for these advancements toward providing integrated mental health services and delivering evidence-backed, innovative treatments tailored to local market needs. NeonMind is a Better Plant portfolio company with an ownership of 26 percent. NeonMind is listed on the CSE under the ticker symbol “NEON”, and on the OTCQB under the ticker symbol “NMDBF”.
About NeonMind Biosciences Inc.
NeonMind operates two divisions: (i) a pharmaceutical division engaged in drug development of psychedelic compounds with two lead psilocybin-based drug candidates targeting obesity; and (ii) a medical services division focused on launching specialty mental health clinics that integrate psychedelic therapeutics into traditional psychotherapy settings.
In its pharmaceutical division, NeonMind has two distinct psilocybin drug development programs targeting obesity. NeonMind’s lead candidate, NEO-001, employs psilocybin as an agonist at the serotonin 5- HT2A receptor, which is involved in the hallucinogenic effect of psychedelics. The Company’s second drug candidate, NEO-002, employs low-dose psilocybin as an agonist at the 5-HT2C receptor, which controls appetite.
NeonMind established a medical services division with the goal of launching NeonMind-branded specialty mental health clinics in Canada that incorporate evidence-backed innovative treatments to address a variety of mental health needs.
For more information on NeonMind, go to www.NeonMindBiosciences.com.
About Better Plant Sciences Inc.
Better Plant harnesses plant intelligence and leverages modern science to offer sustainable, plant-based products that are better for health and better for the earth. It makes and sells over 75 proprietary products, all made with 100% natural ingredients, under the brands Jusu, Urban Juve and Wright & Well. Better Plant operates Jusu Bar, a quick serve restaurant alternative in Victoria, BC, which serves up fresh, healthy, and nutritious options with a focus on Jusu cold-pressed juices. Jusubar.com offers home delivery of refrigerated plant-based beverages consisting of cold-pressed juices and packaged juice cleanses. Through its Shopify enabled eCommerce sites getjusu.com and urbanjuve.com, Better Plant sells plant-based personal care products and cleaning products. Better Plant products are sold through a network of online and brick and mortar retail locations including Whole Foods Market, Pharmasave, Healthy Planet and Vitasave. Better Plant also offers operational, financial, and other services to companies with businesses that align with Better Plant’s mission to help create a better world.
For more information on Better Plant, visit betterplantsciences.com or follow on Instagram, Twitter or LinkedIn.
Penny White, President & CEO
1-833-515-2677
Investor Relations:
Alexandra Dumanski
invest@betterplantsciences.com
1-833-515-2677
Sales Inquiries:
Amber Allen, Head of Sales
604-808-8118
The Canadian Securities Exchange has not reviewed, approved or disapproved the contents of this news release.
Cautionary Statement Regarding Forward-Looking Statements
This press release includes forward-looking information and statements (collectively, “forward looking statements”) under applicable Canadian securities legislation. Forward-looking statements are necessarily based upon a number of estimates, forecasts, beliefs and assumptions that, while considered reasonable, are subject to known and unknown risks, uncertainties, and other factors which may cause actual results and future events to differ materially from those expressed or implied by such forward-looking statements. Such risks, uncertainties and factors include, but are not limited to: risks related to the development, testing, licensing, brand development, availability of packaging, intellectual property protection, reduced global commerce and reduced access to raw materials and other supplies due to the spread of COVID-19, the potential for not acquiring any rights as a result of the patent application and any products making use of the intellectual property may be ineffective or the company may be unsuccessful in commercializing them; and other approvals will be required before commercial exploitation of the intellectual property can happen. Demand for the company’s products, general business, economic, competitive, political and social uncertainties, delay or failure to receive board or regulatory approvals where applicable, and the state of the capital markets. Better Plant cautions readers not to place undue reliance on forward-looking statements provided by Better Plant, as such forward-looking statements are not a guarantee of future results or performance and actual results may differ materially. The forward-looking statements contained in this press release are made as of the date of this press release, and Better Plant expressly disclaims any obligation to update or alter statements containing any forward-looking information, or the factors or assumptions underlying them, whether as a result of new information, future events or otherwise, except as required by law.