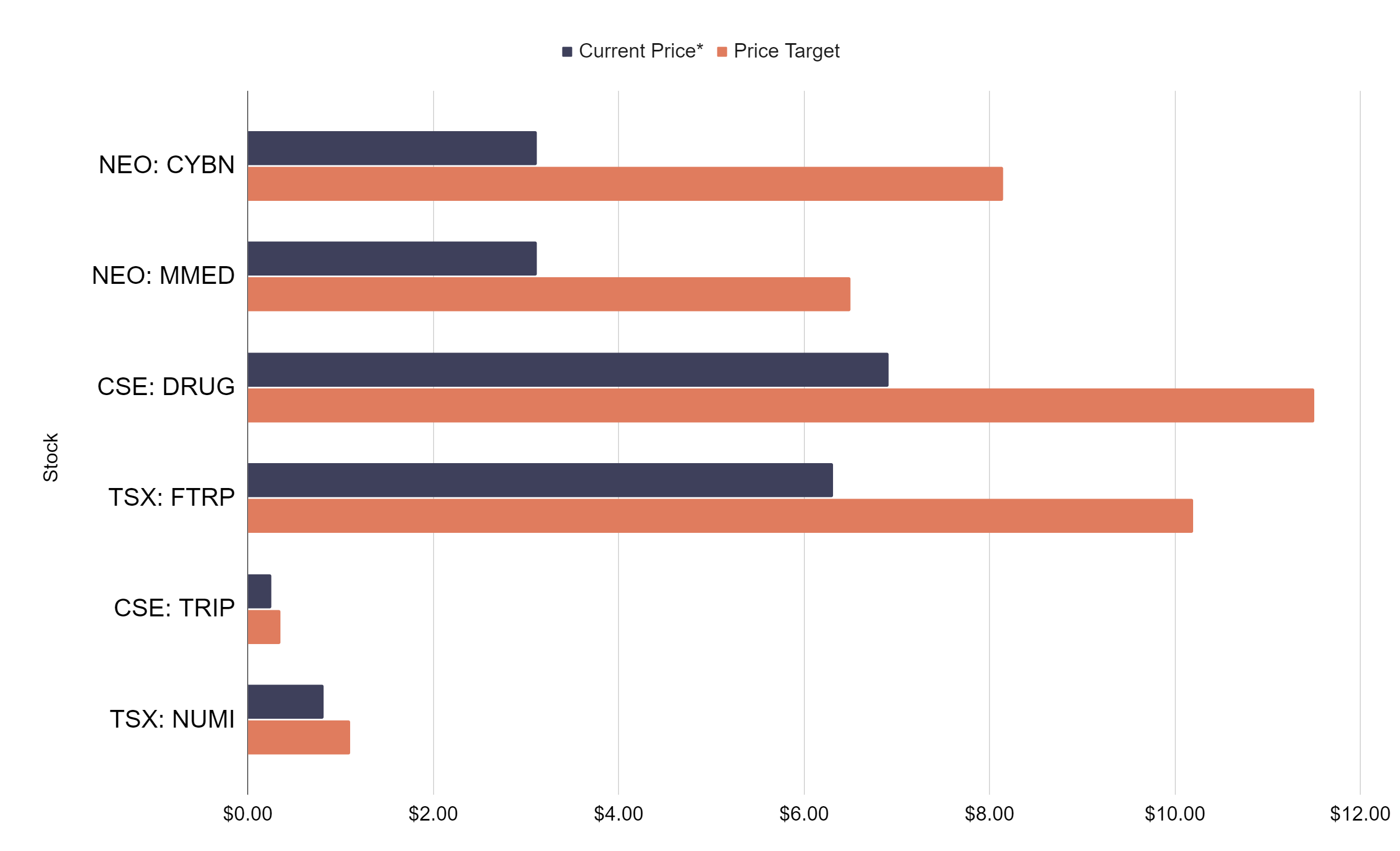
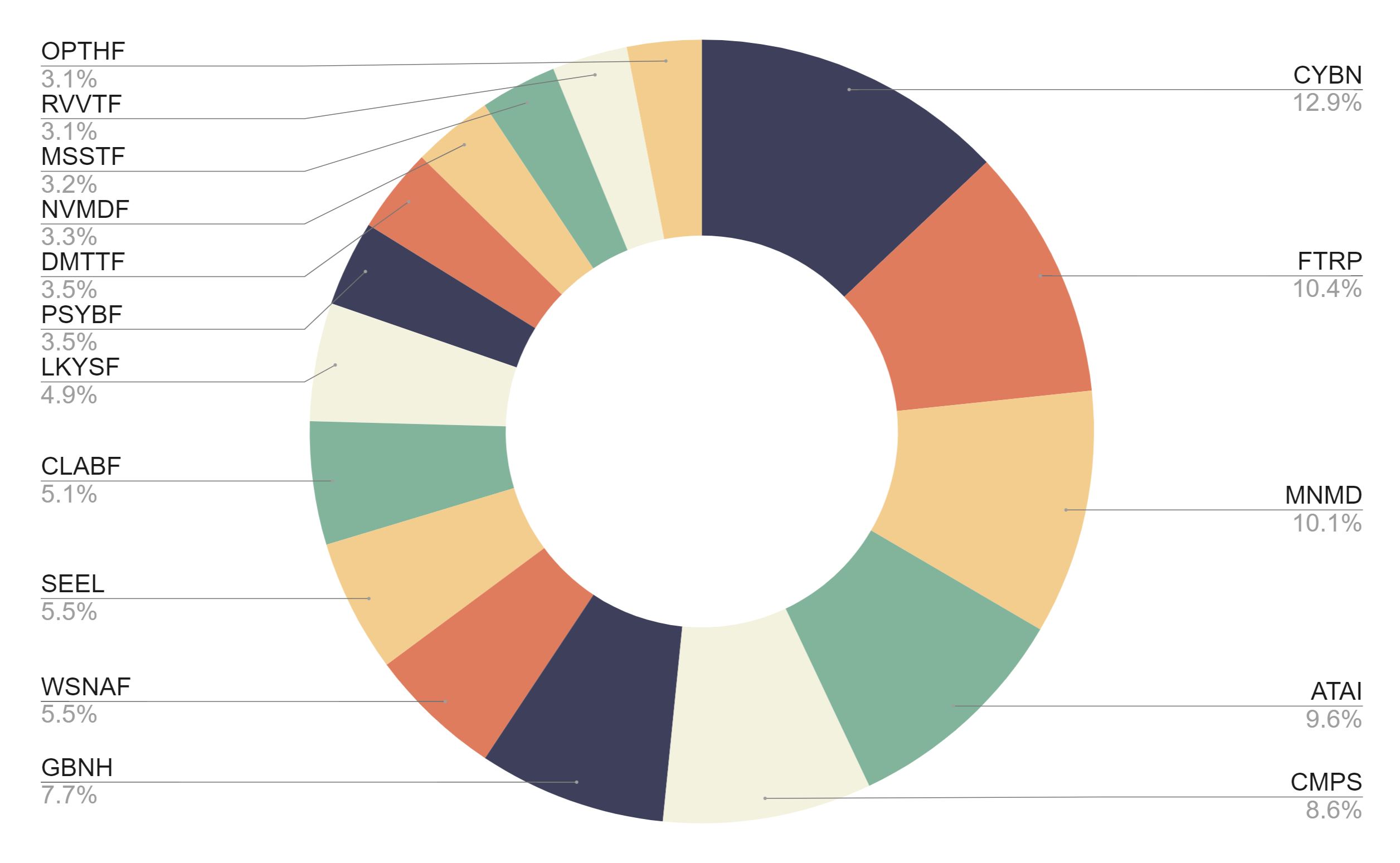
With this acquisition, Enveric will expand and complement its pipeline of naturally occurring cannabinoid compounds with a robust portfolio of psychedelic-derived molecules
Enveric aims to move into the clinic with novel cannabinoid and psychedelic-derived therapies to improve standard of care and serve unmet needs across multiple indications including oncology and CNS, such as PTSD
Upon closing, Dr. Joseph Tucker will be appointed Enveric Biosciences CEO and David Johnson as Executive Chairman
Management will host a conference call to discuss the transaction on Monday, May 24th at 8:30 a.m. ET
NAPLES, Fla., May 24, 2021 /PRNewswire/ — Enveric Biosciences (NASDAQ: ENVB) (“Enveric” or the “Company”), a patient-first biotechnology company developing novel cannabinoid medicines to improve quality of life for cancer patients, today announced that it has entered into a definitive agreement to acquire MagicMed Industries Inc. (“MagicMed”), a privately-held biotechnology company focused on creating a library of novel derivative psychedelic molecules such as psilocybin, N,N-dimethyltryptamine (DMT) and other molecular derivatives with applications across multiple indications, in an all-stock transaction.
Through its extensive R&D capabilities at the state-of-the-art facility at the University of Calgary, MagicMed has focused on the discovery and early development of novel drug candidates, structurally related to psychedelics with vastly improved pharmaceutical characteristics and commercial potential for the treatment of neurological and psychological indications. The Psybrary™ is MagicMed’s library of novel psychedelic derivatives developed through the combination of synthetic biology and traditional chemistry techniques. MagicMed has 13 patent applications filed for derivatives of psilocybin and DMT, 2 patent applications filed for derivatives of mescaline and MDMA with further intellectual property protection for mescaline, MDMA, ibogaine and LSD in process.
The acquisition of MagicMed will expand and complement Enveric’s current pipeline of naturally occurring compounds, which is primarily focused on cannabinoids, to now include a robust portfolio of psychedelic-derived molecules. Enveric intends to continue to develop patient-centric support care therapies in oncology and central nervous system (CNS) indications. Upon closing, Enveric intends to commence drug discovery and development for treatment of cancer-related Post Traumatic Stress Disorder (PTSD) patients who are currently in treatment for cancer and those who are in remission. It is anticipated that the PTSD drug development program holds the potential to be expanded in the future beyond cancer-related applications to include other patient populations, such as military veterans.
“Our proposed acquisition of MagicMed underscores the core fundamental mission of Enveric to form a drug discovery and clinical stage biotechnology company with a focus on bringing forward nature-originated therapies to improve the standard of care and serve unmet needs in oncology and CNS indications,” said David Johnson, Chairman and CEO of Enveric Biosciences. “Psychedelics and cannabinoids, in our opinion, have extensive patient benefits for the mind and body. We welcome MagicMed’s world class research and development team led by Dr. Joseph Tucker, who not only have experience in psychedelic drug discovery, but also research experience in cannabinoids, as well. Patients who are currently being treated for cancer or are in remission are forced to face debilitating physical and mental side effects with very few treatment options available to alleviate their pain. PTSD, for example, is a significant unmet need for this patient population. Together, with the MagicMed team, following the closing of the acquisition, we plan to commence the discovery and development of psychedelics-derived therapies.”
Once closing is complete, Dr. Joseph Tucker will be appointed Chief Executive Officer of the Company and David Johnson, current Chief Executive Officer and Chairman, will be appointed Executive Chairman.
Dr. Joseph Tucker is a seasoned executive who has built several publicly traded biotechnology companies. Dr. Tucker was a founder and chief executive officer of Stem Cell Therapeutics, which was acquired by Trillium Therapeutics in 2013. Dr. Tucker has also held the position of co-founder and CEO of Epimeron Inc., a University of Calgary start-up acquired in the creation of Willow Biosciences Inc. At Willow, Dr. Tucker served as Executive Chairman and COO. Prior to founding these companies, Dr. Tucker was a healthcare analyst with two investment banks and has also worked in technology commercialization for a university technology transfer office. Dr. Tucker received his Ph.D. in Biochemistry and Molecular Biology from the University of Calgary.
“Our mission at MagicMed has always been focused on unlocking the full potential of psychedelic-derived medicines for the treatment of neurological and psychological indications,” added Dr. Joseph Tucker, Chief Executive Officer of MagicMed. “Complementing our R&D capabilities, working together with Enveric will allow us to leverage the company’s clinical team, all of whom are focused on advancing our extensive pipeline of molecules through the clinic to help serve the millions of patients who are suffering with mental health issues around the globe. Through this acquisition – in the best interest of each of our respective teams – we have bolstered our pipeline, bringing together a seasoned team of experts with the leadership skills and knowledge that is crucial to creating a platform that aims to address large unmet total addressable markets (TAM).”
The transaction is structured as an amalgamation under the Business Corporations Act (British Columbia). At the closing, a recently formed subsidiary of Enveric will amalgamate with MagicMed, with the resulting corporation being an indirect wholly owned subsidiary of Enveric. Under the terms of the amalgamation agreement and other related agreements, Enveric will issue the shareholders of MagicMed an aggregate of 9,946,969 shares of common stock of Enveric, as well as warrants, options, and restricted stock units to acquire an additional 9,039,882 shares of common stock of Enveric. The current Enveric shareholders will own approximately 63.4% of the combined company’s common stock, as calculated on a fully diluted basis, and current MagicMed shareholders will own approximately 36.6% of the combined company’s common stock, as calculated on a fully diluted basis. The agreement is subject to customary closing conditions and the approval of Enveric’s and MagicMed’s shareholders and is expected to close during the second half of 2021. Additionally, as part of the closing of the transaction, Enveric will receive approximately $4 million (CAD) in cash from the MagicMed Treasury.
David Johnson and Dr. Joseph Tucker will host a conference call to discuss the transaction today, May 24th at 8:30 a.m. ET.
Conference Call Details:
Date: Monday, May 24th
Time: 8:30 a.m. Eastern Time
Toll-Free Dial-In Number: 1-877-705-6003
International Dial-In Number: 1-201-493-6725
Conference ID: 13720013
Webcast Link: https://www.enveric.com/investors/events/
A telephone replay will be available through Monday, June 7, 2021. To access the replay, please dial 1-844-512-2921 (domestic) or 1-412-317-6617 (international). At the system prompt, please enter the code 13720013 followed by the # sign.
About Enveric Biosciences
Enveric Biosciences is a patient-first biotechnology company developing rigorously tested, novel cannabinoid medicines to improve quality of life for cancer patients. Initial indications include radiodermatitis, a common and often severe side effect of radiation therapy, and chemotherapy-induced neuropathy. For more information, please visit https://www.enveric.com/.
About MagicMed
MagicMed Industries intends to partner with pharmaceutical and other companies to develop and commercialize psychedelic-derived pharmaceutical candidates. MagicMed’s psychedelic derivatives library, the Psybrary™, is anticipated to be an essential building block from which industry can develop new patented products. The initial focus of the Psybrary™ is on psilocybin and DMT derivatives, and it is then expected to be expanded to other psychedelics such as MDMA, LSD, mescaline, and ibogaine.
No Offer or Solicitation
This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No public offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.
Important Additional Information Will be Filed with the SEC
In connection with the proposed transaction between Enveric and MagicMed, Enveric intends to file relevant materials with the SEC, including a registration statement that will contain a proxy statement and prospectus. ENVERIC URGES INVESTORS AND STOCKHOLDERS TO READ THESE MATERIALS CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT ENVERIC, THE PROPOSED TRANSACTION AND RELATED MATTERS. Investors and shareholders will be able to obtain free copies of the proxy statement, prospectus and other documents filed by Enveric with the SEC (when they become available) through the website maintained by the SEC at www.sec.gov. In addition, investors and shareholders will be able to obtain free copies of the proxy statement, prospectus and other documents filed by Enveric with the SEC by contacting Investor Relations by mail at Enveric Biosciences, Inc., Attn: Investor Relations, 4851 Tamiami Trail N, Suite 200, Naples, FL 34103. Investors and stockholders are urged to read the proxy statement, prospectus and the other relevant materials when they become available before making any voting or investment decision with respect to the proposed transaction.
Participants in the Solicitation
Enveric and MagicMed, and each of their respective directors and executive officers and certain of their other members of management and employees, may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about Enveric’s directors and executive officers is included in Enveric’s Annual Report on Form 10-K for the year ended December 31, 2020, filed with the SEC on April 1, 2021. Additional information regarding these persons and their interests in the transaction will be included in the proxy statement relating to the transaction when it is filed with the SEC. These documents can be obtained free of charge from the sources indicated above.
Cautionary Statement Regarding Forward-Looking Statements
This communication contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. You can generally identify forward-looking statements by the use of forward-looking terminology such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “explore,” “evaluate,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “seek,” “should,” or “will,” or the negative thereof or other variations thereon or comparable terminology. These forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond Enveric’s and MagicMed’s control. Statements in this communication regarding Enveric, MagicMed and the combined company that are forward-looking, including projections as to the anticipated benefits of the proposed transaction, the impact of the proposed transaction on Enveric’s and MagicMed’s business and future financial and operating results, the amount and timing of synergies from the proposed transaction, expectations regarding capital structure following the closing of the proposed transaction, the combined company’s pipeline, intellectual property protection and R&D spend, and the closing date for the proposed transaction, are based on management’s estimates, assumptions and projections, and are subject to significant uncertainties and other factors, many of which are beyond Enveric’s and MagicMed’s control. These factors include, among other things, the combined company’s ability to execute successfully its strategic plans, including its business development strategy, the expiration of patents or data protection on certain products, including assumptions about the combined company’s ability to retain patent exclusivity of certain products, the impact and result of governmental investigations, the combined company’s ability to obtain necessary regulatory approvals or obtaining these without delay, the risk that the combined company’s products prove to be commercially successful or that contractual milestones will be achieved. Similarly, there are uncertainties relating to a number of other important factors, including: results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the U.S. FDA and other regulatory authorities, investigational review boards at clinical trial sites and publication review bodies; the ability to enroll patients in planned clinical trials; unplanned cash requirements and expenditures; the amount of funds the combined company requires for its product candidates; competitive factors; the ability to obtain, maintain and enforce patent and other intellectual property protection for any product candidates; the ability to maintain key collaborations; the impact of the ongoing COVID-19 pandemic on combined company’s results of operations, business plan and the global economy; and general economic and market conditions. Additional information concerning these risks, uncertainties and assumptions can be found in Enveric’s filings with the SEC, including the risk factors discussed in Enveric’s most recent Annual Reports on Form 10-K, as updated by its Quarterly Reports on Form 10-Q and future filings with the SEC.
Important risk factors could cause actual future results and other future events to differ materially from those currently estimated by management, including, but not limited to, the risks that: a condition to the closing of the proposed acquisition may not be satisfied; a regulatory approval that may be required for the proposed acquisition is delayed, is not obtained or is obtained subject to conditions that are not anticipated; Enveric is unable to achieve the synergies and value creation contemplated by the proposed acquisition; Enveric is unable to promptly and effectively integrate MagicMed’s businesses; management’s time and attention is diverted on transaction-related issues; disruption from the transaction makes it more difficult to maintain business, contractual and operational relationships; legal proceedings are instituted against Enveric, MagicMed or the combined company; Enveric, MagicMed or the combined company is unable to retain key personnel; and the announcement or the consummation of the proposed acquisition has a negative effect on the market price of the capital stock of Enveric. No assurances can be given that any of the events anticipated by the forward-looking statements will transpire or occur, or if any of them do occur, what impact they will have on the results of operations or financial condition of Enveric or MagicMed. Should any risks and uncertainties develop into actual events, these developments could have a material adverse effect on the proposed transaction and/or Enveric or MagicMed, Enveric’s ability to successfully complete the proposed transaction and/or realize the expected benefits from the proposed transaction. You are cautioned not to rely on Enveric’s and MagicMed’s forward-looking statements. These forward-looking statements are and will be based upon management’s then-current views and assumptions regarding future events and operating performance, and are applicable only as of the dates of such statements. Neither Enveric nor MagicMed assumes any duty to update or revise forward-looking statements, whether as a result of new information, future events or otherwise, as of any future date.
Investor Contacts
Valter Pinto / Allison Soss
KCSA Strategic Communications
212.896.1254 / 212.896.1267
valter@kcsa.com / asoss@kcsa.com
Media Contacts
Caitlin Kasunich / Raquel Cona
KCSA Strategic Communications
212.896.1241 / 516.779.2630
ckasunich@kcsa.com / rcona@kcsa.com
SOURCE Enveric Biosciences
![]() View original content:https://www.prnewswire.com/news-releases/enveric-biosciences-to-participate-in-upcoming-investor-conferences-in-september-2021-301380168.html
View original content:https://www.prnewswire.com/news-releases/enveric-biosciences-to-participate-in-upcoming-investor-conferences-in-september-2021-301380168.html